Cancer Research Advocates Committee
Purpose
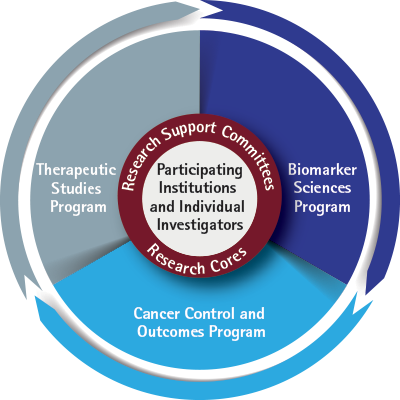
The Cancer Research Advocates Committee (CRAC) is a standing committee that brings the patient perspective and experience to the cancer clinical trials being designed and conducted at the ECOG-ACRIN Cancer Research Group (ECOG-ACRIN). CRAC members—all cancer survivors and caregivers, offer unique patient experiences and connections to their networks of cancer support organizations, patients, and other advocates for bi-directional communication.
Goals/Specific Aims
ECOG-ACRIN research advocates are active members of committees across the Scientific Programs, participating in regular meetings and conference calls. They are a resource in multiple ways, advising research teams as they discuss promising new scientific ideas and plan new clinical trials.
There are four essential time points when research advocates represent the needs and concerns of patients:
- Design stage – Explain the practical aspects of being in a clinical trial and what might be attractive or a barrier for potential participants
- Protocol and informed consent document review – Review the informed consent document, focusing on whether it is understandable to a lay audience, using the protocol document as a reference
- Trial activation – Review the patient education materials for whether they are understandable to patients (in simple language and free from acronyms or technical medical terms), and assess recruitment and social media plans for their reach into the patient community
- Results reporting – Ensure that advocacy organizations and patients learn about clinical trial results that lead to improvements or changes in cancer treatment, and published results of clinical trials are summarized in language that is understandable to a lay audience. These summaries are currently available in ECOG-ACRIN’s quarterly Advocacy Blog.
Members and Their Committee Assignments
Chair
Represents research advocacy on the Executive Committee and NCORP Community Advisory Committee. Also serves as the research advocate on the Breast Cancer Committee and Cancer Control and Survivorship Committee.
- Cancer Control and Survivorship Committee; Cardiotoxicity Working Group
Mitch Achee
- Sarcoma Working Group
Carole Baas
- Breast Cancer Committee; Experimental Imaging Science Working Group
Melinda Bachini
- Gastrointestinal Cancer Committee
Lorna Beccaria
- Developmental Therapeutics Committee; NCI-MATCH Trial; Laboratory Science and Pathology Committee
Lisa Beckendorf
- Leukemia Committee
Yelak Biru
- Myeloma Committee
Steven Buechler
- Leukemia Committee
Stephanie Chisolm
- Genitourinary Committee
Trish Cosentino
- Lymphoma Committee
Arlene Dahm
- Gastrointestinal Cancer Committee
Robin Evans
- Breast Cancer Committee; Prevention, Screening and Surveillance Committee
Jill Feldman
- Thoracic Cancer Committee
Lauren Ghazal, PhD, FNP-BC
- Adolescent and Young Adult Oncology Subcommittee (part of the Health Equity Committee)
Sam Guild
- Melanoma Committee
Lucy Hanna
- Committee affiliations to come
Brenda Hopper
- Breast Cancer Committee
Sanford Jeames
- Cancer Control and Survivorship Committee; Gastrointestinal Cancer Committee; Prevention, Screening and Surveillance Committee
Brian Jones
- Genitourinary Cancer Committee
Wayne Keese
- Leukemia Committee
Neal Levitan
- Brain Tumor Working Group
Debra Madden
- Breast Cancer Committee; Cancer Control and Survivorship Committee
Steven Merlin
- Gastrointestinal Cancer Committee
Anne Patterson
- Cancer Control and Survivorship Committee
Glenn Sykes
- Genitourinary Committee